Pressure, Volume, and Density Relationships
Pressure is one of the most important physical factors to understand as a scuba diver. The pressure on you underwater consists of two forces that act together: the weight of the water above you and the weight of the surface atmosphere acting on the surface of the water.
You do not feel much pressure underwater because you are made up mostly of liquids—mostly of water, in fact—and unlike air and other diving gases, water does not compress. However, you will feel the changes in pressure as the weight of water at depth exerts pressure on gas cavities, such as your sinuses, your middle ears, and the air space in your mask.
Pressure can be expressed in pounds per square inch(PSI), bar, or atmospheres. At sea level, the pressure is 1 atmosphere (ata), which is approximately equal to 1 bar or 14.7 psi or 760 mm Hg (mercury). You are used to living at 1 atmosphere (ata) of pressure, so you rarely take notice of it. You will notice changes of pressure in your ears when swimming deeper, flying, or driving up in the mountains. This is because your ears and sinuses have air spaces in them; therefore, they are compressible. The gas will compress or expand proportionately to the changes in the pressure exerted on it.
Gas can be compressed. This is wonderful for divers because breathing gases can be squeezed into the diving cylinders so that divers can take a huge volume of gas on their dives in a much smaller container. Divers need to understand that as gas is compressed into less space, the pressure inside the space—a scuba cylinder, for example—increases proportionally. This relationship is described by Boyle’s Law.
A potential pressure-related problem comes when you consider your lungs. If you were to take a lungful of compressed gas at depth and were to hold your breath and start to surface, your lungs would overexpand and rupture as the air expanded. This is why divers are told from the very first scuba lesson to never hold their breath when diving. Boyle’s Law is behind the mechanics of lung overexpansion.
Let’s say a diver takes a full breath of compressed gas at 10 meters/33 feet/2 bar. There would be twice as much pressure on his or her body at that depth as there is on the surface. To overcome that increased pressure and to fill the lungs, the gas would have to be twice the density. Scuba regulators help a diver to do this.
As the absolute pressure in the water (the ambient pressure) increases or decreases as you descend or ascend, the density of gas in your lungs increases or decreases proportionately. This means that if pressure increases, the density of gas in your lungs increases. And so, you have to breathe out to expel the expanding “extra” gas as you surface. Grasping this concept is vitally important to you when you are diving.

Right: A scuba diver breathes more air on descent to keep the lungs full but must exhale on ascent to let the expanding gas escape.
Effects of Pressure, Volume, and Density on Scuba Diving
Table 1 below shows the balance between gas volume and gas density in a flexible container, like a lung. As the pressure increases with depth, the volume will decrease, and the density will increase. This is what happens with any gas space in your body and equipment, for example, your ears, sinuses, mask, and lungs.

Table 2 shows that even though a diver’s lungs stay the same size at depth (the same volume), the volume of gas needed to fill them increases proportionally as depth increases (Boyle’s Law in action). This is because as the ambient pressure surrounding a diver increases, the air in her or his lungs has to increase as well. Increased pressure means the gas is denser, and denser gas means more gas. Therefore, as a diver ventures deeper, he or she consumes more gas per breath.

As absolute pressure decreases during ASCENT, the gas volume in your lungs will increase proportionately. So, the volume of gas expands. If a diver should hold his or her breath at 10 meters/33 feet and ascend with 8 liters of compressed gas inside lungs that can hold only 4 liters (as in Table 1), the expanding gas would cause serious damage to the diver’s lungs.
Have you noticed anything? The biggest pressure and gas volume change is between 10 meters/33 feet and the surface. The volume of gas doubles on ascent or halves on descent. In only a couple of meters or a few feet, the pressure differential is large. Because of the rapid change in just 10 meters/33 feet, this is the area where you have to be the most cautious, especially with regard to barotraumas (lung overexpansion), which can occur when you hold your breath.
Remember…
Never hold your breath when scuba diving. Move up and down slowly, and do your safety stop on ascent.
we’re going to cover six important diving laws and principles that every diver needs to be aware of. We urge you to study this article and develop a full understanding of how these laws work and to draw your own opinions about why they’re important.
Boyle’s Law
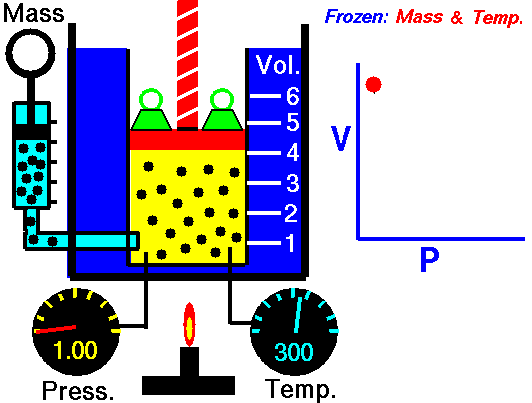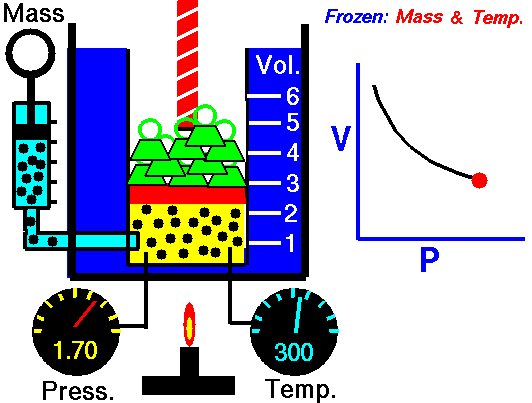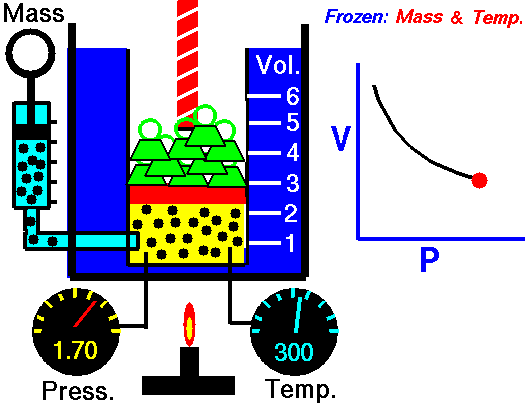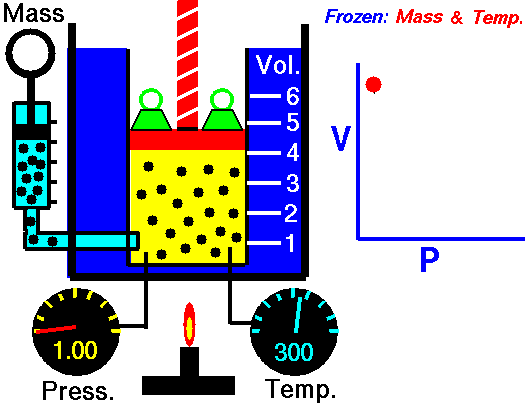
Boyle’s Law states that “given a constant temperature, the volume of a gas varies inversely as the absolute pressure.”
That means that as the pressure increases, the volume decreases. As pressure decreases, the volume will increase.
To illustrate this law, we take a bottle full of air down to a depth of about 100ft (30m), 3 ATM (atmospheres gauge) or 4 ATA (atmospheres absolute). As the tank descends, the absolute pressure around the tank forces the gas (Air) inside the tank to compress therefore allowing for less volume. This happens because as you go deeper into water, the pressure increases at a rate of one ATM every 33ft (10m). This is the pressure that decreases the volume of gas inside your tank.
Lets say we take that tank up into the air 36,000 ft. (10,970m), cruising altitude for a standard commercial jet liner. The air in the atmosphere actually has a lower absolute pressure than we do here on the surface. Now, using Boyle’s Law we can conclude that, air in the tank will actually increase in volume as the absolute pressure around it decreases.
P1/V1=P2/V2
Charles’s Law / Gay-Lussac’s Law
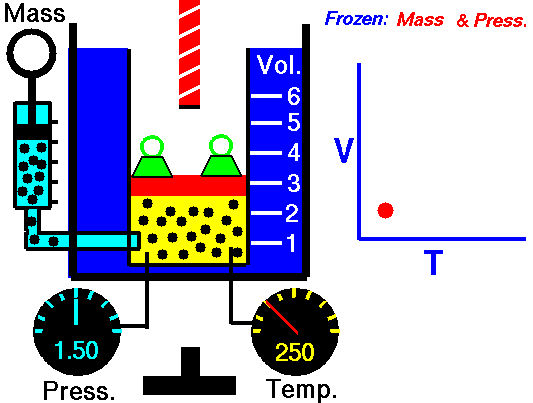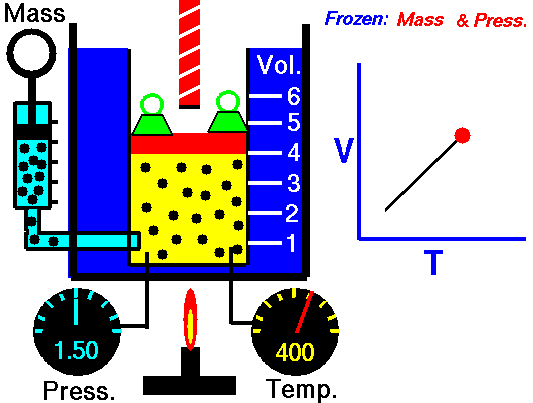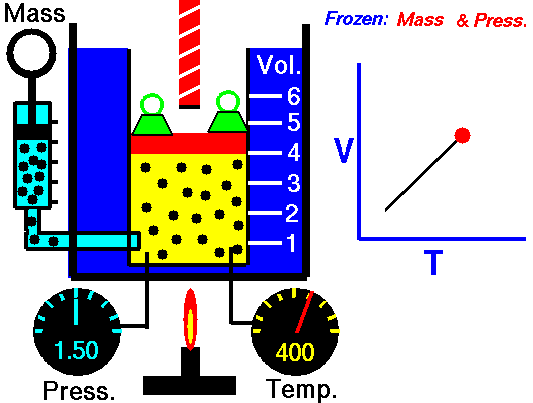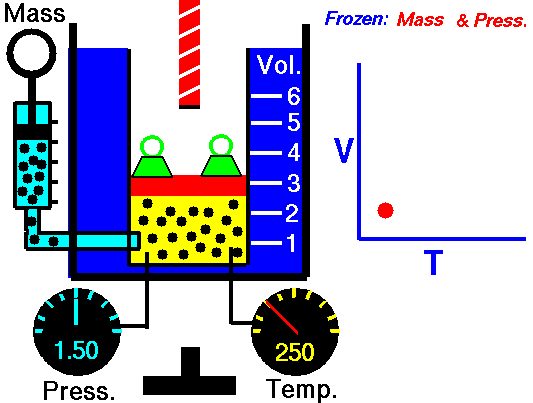
Charles’s law states that given a constant pressure, the volume of a gas varies directly as the absolute temperature. This law is sometimes referred to as Gay-Lussac’s Law.
This is probably one of the simpler laws. Most of you know that as metal gets hot or cold, it expands and contracts (more notably than other things like plastic). Well gases do the same thing.
What Charles’s Law says is that if you take a volume of air, and keep it at a constant pressure, you can increase your volume simply by heating it up. You could also effectively reduce the volume by putting it in a deep freeze, or setting it outside if you live up north. So to illustrate this law, we use a SCUBA tank.
When a SCUBA cylinder is filled, the friction of the air entering the cylinder heats things up from the inside out. This makes the surface of the cylinder warm to the touch. Just about all of your shops will overfill cylinders by about 10%, which is the maximum allowed by the DOT. There is a reason for this.
Every cylinder model has a different “working pressure,” which means that they can handle being filled to lets say 3000 psi (206 bar), plus 10% of that pressure. So then we have a cylinder that gets filled to 3300 psi (227 bar). This is not a safe working pressure for the cylinder (theoretically), so why do we fill a cylinder to more than its safe working pressure? They are overfilled because of Charle’s Law. Friction heats the cylinder and that cylinder will eventually cool off, thus, decreasing the volume of air inside. So to counter this effect, dive shops put more air in the cylinder than would be necessary for a typical dive.
Dalton’s Law
Dalton’s Law states that the total pressure exerted by a mixture of gases is the sum of the pressures exerted by each of the gases.
This is pretty straight forward. It becomes most useful for anyone qualified to fill a scuba cylinder. Lets say your target working pressure is 3000 psi (206 bar) for a typical SCUBA cylinder fill. Normally, you would take the air from the room you are standing in, compress it down, and pump it into a cylinder.
The air we breath is an average 21% Oxygen 79% Nitrogen. Nitrox is a gas with an oxygen content of 22%-40%.
Many factors go into consideration, such as working pressure, cylinder volume, percent of O2 desired, etc. The basic formula says this: start with Xpsi of 100% O2, and then fill to 3000 psi (206 bar) with air. This gives the desired percentage of oxygen in your nitrox gas. So we have a hypothetical 400 psi (27 bar) of pure oxygen, and we will top off the cylinder with 2600 psi (179 bar) with air, leaving 3000 psi (206 bar) of pressure in the cylinder. 400 + 2600= 3000psi (27+179=206 bar). The total pressure is equal to the sum of the pressure of each part of the mixture.
Henry’s Law
Henry’s Law states that the amount of gas that will dissolve in a liquid is directly proportional to the partial pressure of that gas.
Here is a law that goes much deeper than mixing gases and heating SCUBA cylinders.
This is the mother of dive laws.
If you’ve taken a diving class, you know that nitrogen is your biggest opponent underwater. Nitrogen happens to be a gas, You see, while you’re on the surface, nitrogen absorbs into your skin. This is not a real issue while you’re walking around, driving your cars, etc. Take that body down to 100 ft (30 m) of water and you change the circumstances dramatically.
During your time underwater, the pressure around your body forces nitrogen into your blood stream. This can (but not always) lead to a rather pleasant narcotic feeling at depth we refer to as “nitrogen narcosis.” Nitrogen has a narcotic effect on the brain which can be alleviated by ascending to a shallower depth until symptoms pass. The longer you stay, and the deeper you go, the more nitrogen will be forced into your blood stream. If you don’t get narcosis easily, then you will most likely not notice the build up of excess nitrogen in your blood.
The nitrogen at depth is not the issue, but rather when you complete your dive and begin your ascent to the surface. As you ascend, the nitrogen gas that has dissolved into your blood stream will begin to form bubbles as the gas expands. If you were to acquire an excess of nitrogen and immediately get out of the water, within about 15 minutes you would begin showing symptoms of DCS (decompression sickness) which can be fatal if not treated. This sickness has been dubbed “the bends,” or “Cassien’s disease” by divers in the past. This build up of nitrogen is removed from the body by ascending to a shallower depth and allowing the nitrogen to pass through your blood stream, and into your lungs. Once in your lungs, the nitrogen returns to its undissolved, gas state and passes from your body as you exhale. The depth and amount of time required for “off-gassing” is directly related to the amount of time, and depth of your dive.
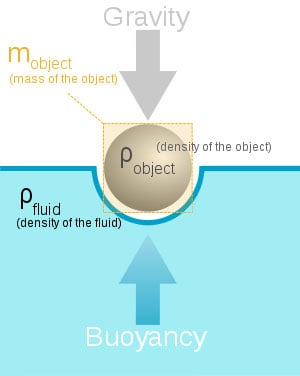
Archimedes Principle
Archimedes Principle states that and object wholly or partially immersed in a liquid is buoyed up by the force equal to the weight of the liquid displaced.
This one is very simple to understand. Lets say we fill a cup with water until it’s about to spill over the edge. Then we take a rock, and drop it in that cup, the water would begin to flow over onto the table. Now to find the value referenced by Archimedes Principle, we would simple take the water that spilled, and weigh it. Lets say for the sake of argument the displacement of the water weighs eight grams. That means, according to the principle in discussion, that the rock is being pushed back up with a force of 8 grams.
We use this law for many different things while in the water. It is mostly used to calculate the specific buoyancy of an object. This will be important for anyone that spends time underwater. For instance, if your Buoyancy Compensation Device does not have the lift capacity to bring up your steel SCUBA cylinders, you will surely be stuck on the bottom. We can set values to plan these things because of Archimedes Principle.
This law doesn’t only apply to the underwater community. Naval Architects will use this principal to calculate many different requirements of a ship’s hull. An aircraft carrier is made mostly out of steel, but because of the way she’s designed, she floats. This is the issue with ships as well, if we use Archimedes Principle along with other amazingly simple laws, we have enormous metal structures floating and moving under their own power.